Identify the spectator ions in the following molecular equation – In the realm of chemistry, identifying spectator ions in molecular equations is a fundamental skill that unveils the intricate details of chemical reactions. Spectator ions, like silent observers, remain unchanged throughout the reaction, providing valuable insights into the behavior of the participating species.
This comprehensive guide delves into the concept of spectator ions, exploring their significance and providing a step-by-step approach to their identification, empowering you to decipher complex molecular equations with precision.
As we embark on this journey, we will uncover the practical applications of identifying spectator ions, enabling you to simplify chemical calculations and make accurate predictions. By the end of this exploration, you will possess a profound understanding of spectator ions, their role in chemical reactions, and their indispensable contribution to the field of chemistry.
Spectator Ions in Molecular Equations
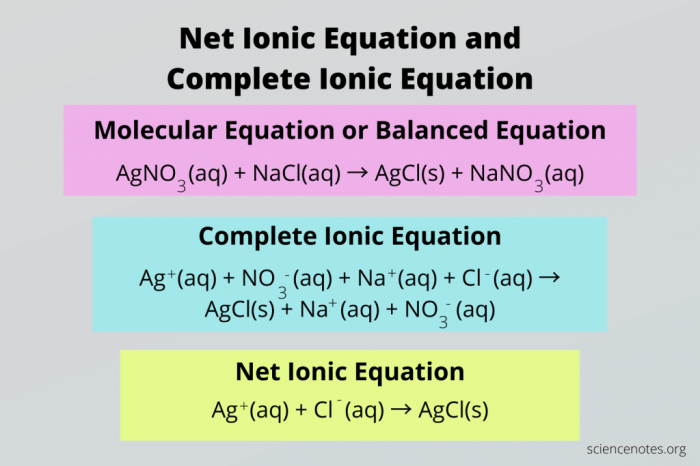
In chemistry, a spectator ion is an ion that does not participate in a chemical reaction. Spectator ions are present on both sides of a chemical equation and do not undergo any change during the reaction. Identifying spectator ions is important because it allows us to write net ionic equations, which are equations that show only the ions that participate in the reaction.
Identifying Spectator Ions

To identify spectator ions, we must first understand the concept of net ionic equations. A net ionic equation is an equation that shows only the ions that participate in a chemical reaction. To write a net ionic equation, we must first balance the molecular equation.
Once the molecular equation is balanced, we can then identify the spectator ions. Spectator ions are the ions that appear on both sides of the molecular equation and do not undergo any change during the reaction.
Step-by-Step Process for Identifying Spectator Ions, Identify the spectator ions in the following molecular equation
- Balance the molecular equation.
- Write the ionic equation by separating the reactants and products into their respective ions.
- Identify the ions that appear on both sides of the ionic equation. These are the spectator ions.
- Remove the spectator ions from the ionic equation to obtain the net ionic equation.
Examples of Spectator Ions
Here are some examples of molecular equations and the spectator ions:
| Molecular Equation | Spectator Ions |
|---|---|
| NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq) | Na+ and NO3– |
| CuSO4(aq) + Ba(OH)2(aq) → Cu(OH)2(s) + BaSO4(s) | SO42- and Ba2+ |
| FeCl3(aq) + NaOH(aq) → Fe(OH)3(s) + NaCl(aq) | Na+ and Cl– |
Applications of Identifying Spectator Ions: Identify The Spectator Ions In The Following Molecular Equation
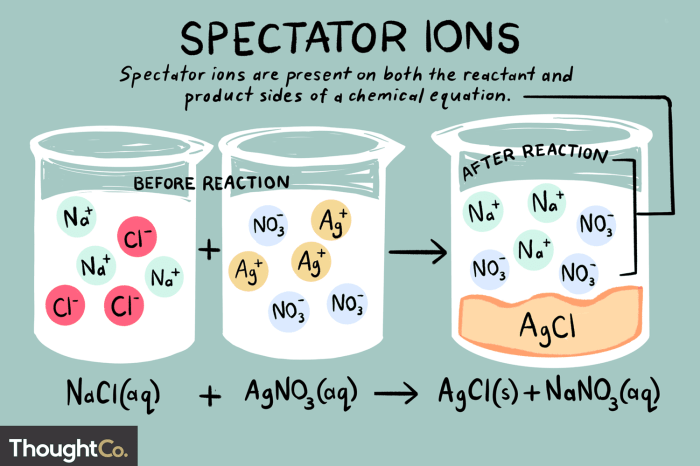
Identifying spectator ions has several practical applications. One application is that it can simplify chemical calculations. For example, if we want to calculate the mass of a precipitate that will form in a reaction, we can use the net ionic equation to determine the moles of the precipitate that will form.
Another application of identifying spectator ions is that it can help us to predict the products of a reaction. For example, if we know the spectator ions in a reaction, we can predict the ions that will form the precipitate.
Additional Considerations
There are some exceptions to the rules for identifying spectator ions. For example, in some cases, an ion may appear on both sides of a molecular equation but still participate in the reaction. This can occur if the ion is involved in an equilibrium reaction.
Another exception is that in some cases, an ion may appear on both sides of a molecular equation but still undergo a change in oxidation state. This can occur if the ion is involved in a redox reaction.
Essential FAQs
What is the significance of identifying spectator ions in molecular equations?
Identifying spectator ions allows us to focus on the essential chemical changes occurring in a reaction, simplifying calculations and providing a clearer understanding of the reaction’s mechanism.
How do spectator ions affect the net ionic equation of a reaction?
Spectator ions do not participate in the chemical reaction and, therefore, do not appear in the net ionic equation, which represents only the essential chemical changes.
Are there any exceptions to the rules for identifying spectator ions?
Yes, there are some exceptions, such as when ions form complex ions or participate in redox reactions. These exceptions require a deeper understanding of chemical principles.